Publications
Prevalence and characterization of ESR1 alterations detected with an ultra-sensitive, liquid-only CGP assay in a large breast cancer cohort
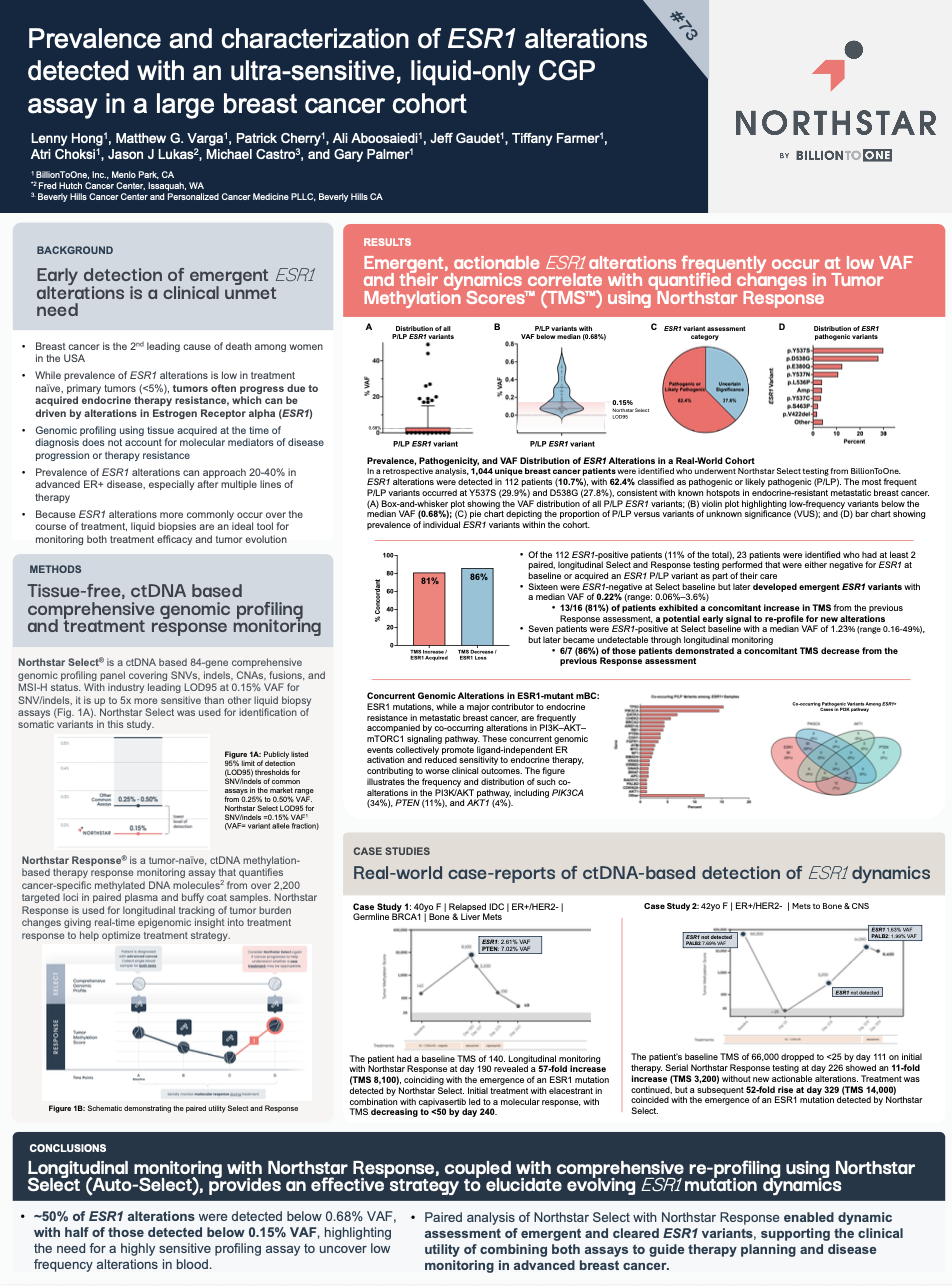
Prevalence and characterization of ESR1 alterations detected with an ultra-sensitive, liquid-only CGP assay in a large breast cancer cohort

Validation of a liquid biopsy assay with increased sensitivity for clinical comprehensive genomic profiling

Validation of a liquid biopsy assay with increased sensitivity for clinical comprehensive genomic profiling

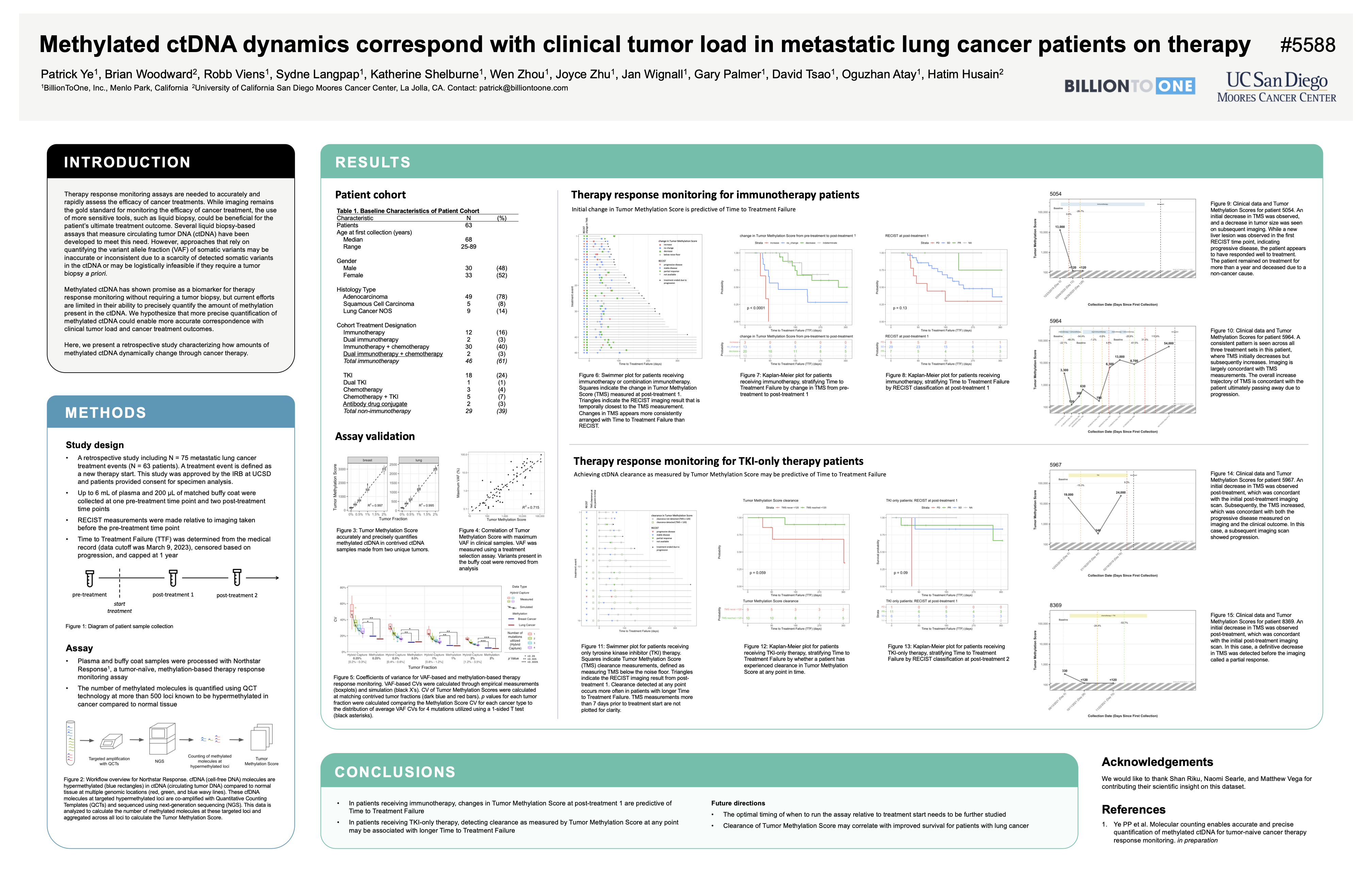
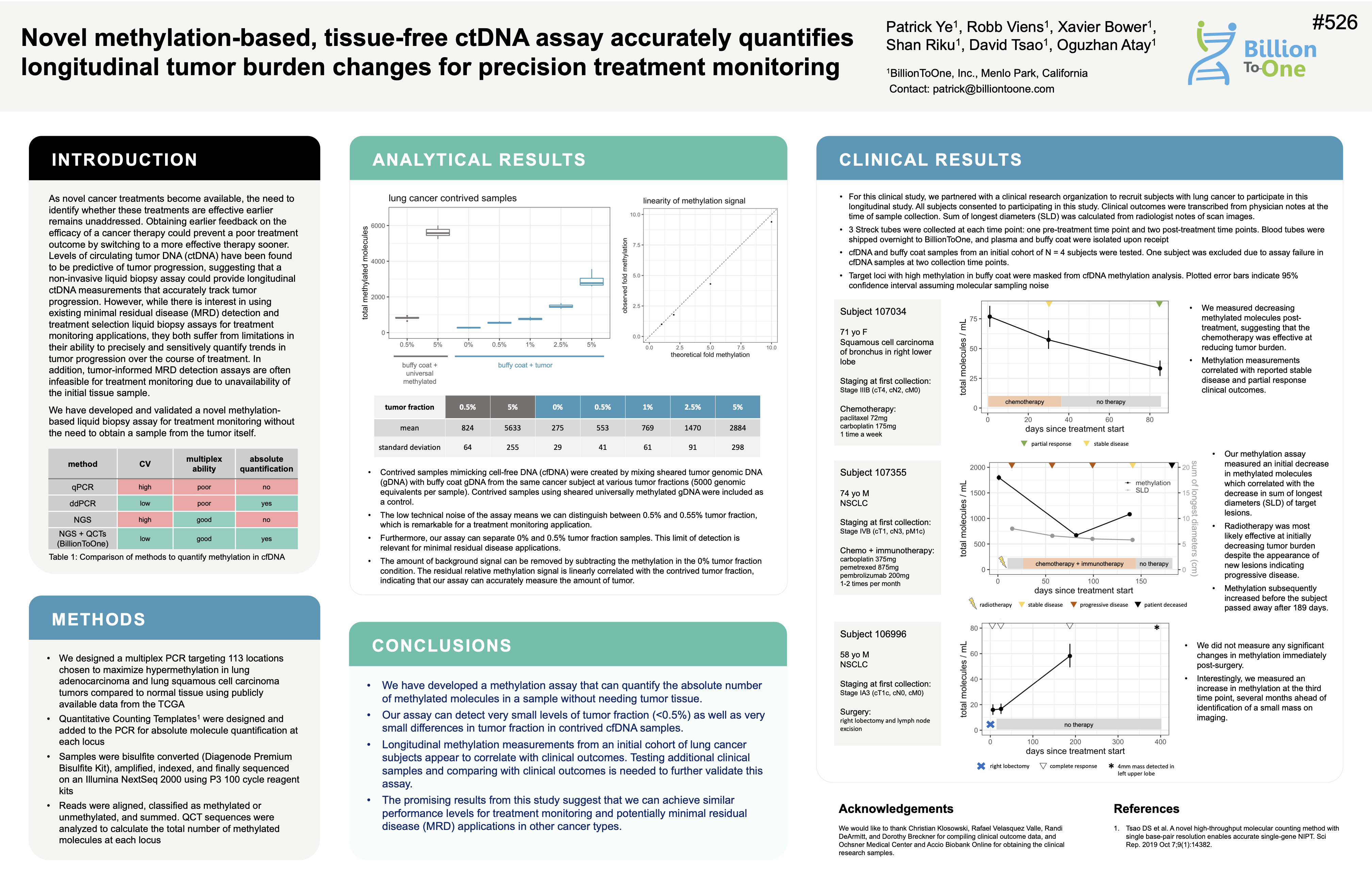
Circulating cell-free methylated tumor DNA measurements correlate with plasma VAF-based tumor fraction estimates

Circulating cell-free methylated tumor DNA measurements correlate with plasma VAF-based tumor fraction estimates

Detection of a novel GNA11 processed pseudogene in chromosome 20 from cfDNA and implications for liquid biopsy

Detection of a novel GNA11 processed pseudogene in chromosome 20 from cfDNA and implications for liquid biopsy

Tumor fraction estimation and tissue copy number inference using copy number signal from a liquid biopsy assay

Tumor fraction estimation and tissue copy number inference using copy number signal from a liquid biopsy assay

Molecular counting enables accurate and precise quantification of methylated ctDNA for tumor-naive cancer therapy response monitoring
Molecular counting enables accurate and precise quantification of methylated ctDNA for tumor-naive cancer therapy response monitoring

Clinical Validation of the Northstar Response: A Novel Quantitative Methylation ctDNA Monitoring Assay for Advanced GI Cancer Treatment Response
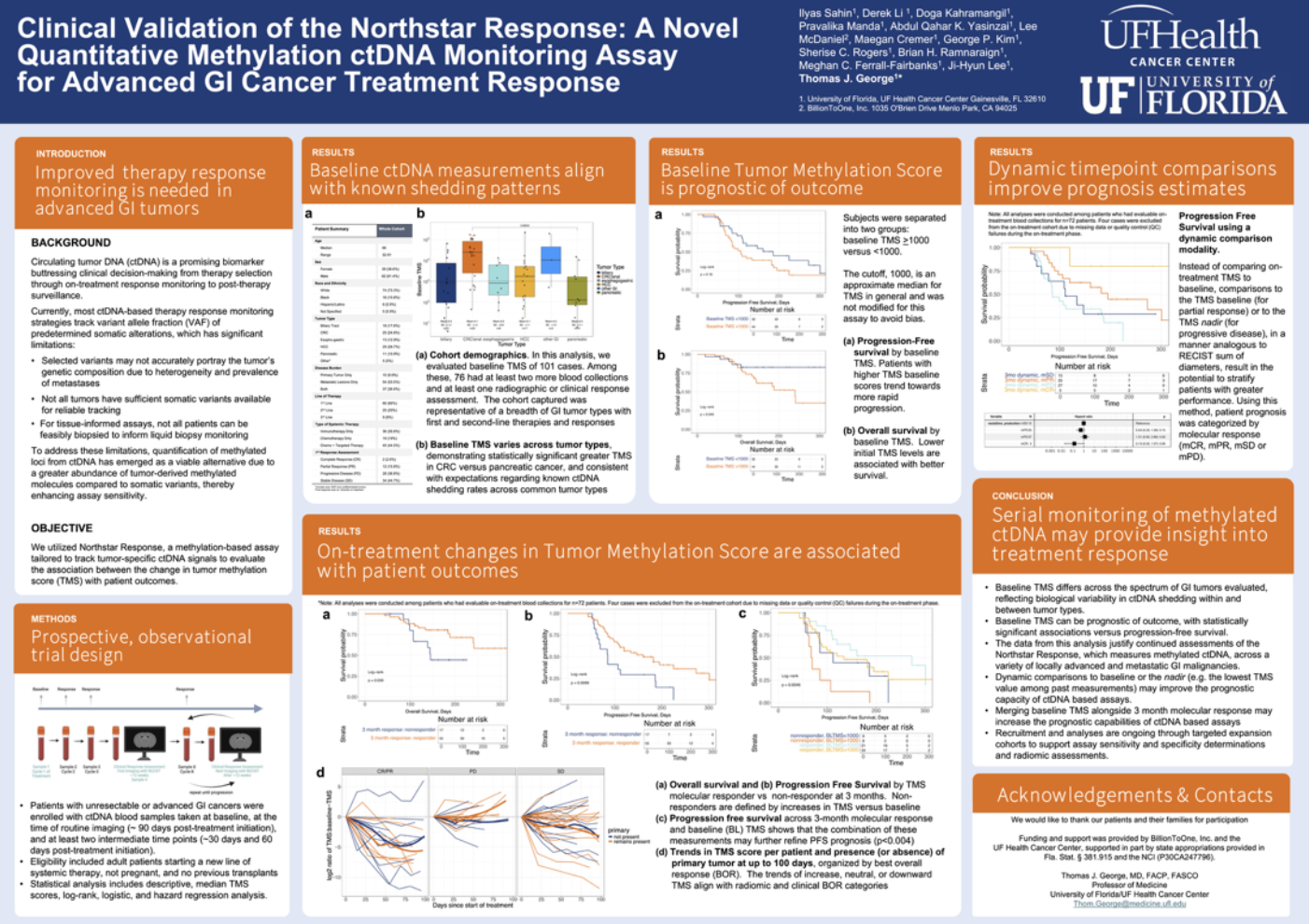
Clinical Validation of the Northstar Response: A Novel Quantitative Methylation ctDNA Monitoring Assay for Advanced GI Cancer Treatment Response

Absolute quantification of cell-free DNA for prenatal genetics and oncology

Absolute quantification of cell-free DNA for prenatal genetics and oncology

Brief Report: Methylation-Based ctDNA Serial Monitoring Correlates With Immunotherapy Response in NSCLC

Brief Report: Methylation-Based ctDNA Serial Monitoring Correlates With Immunotherapy Response in NSCLC

Methylation-Based ctDNA Serial Monitoring Correlates with Immunotherapy Response in NSCLC

Methylation-Based ctDNA Serial Monitoring Correlates with Immunotherapy Response in NSCLC

A novel liquid biopsy assay with an 88% detection rate of genomic alterations across CNS tumors.
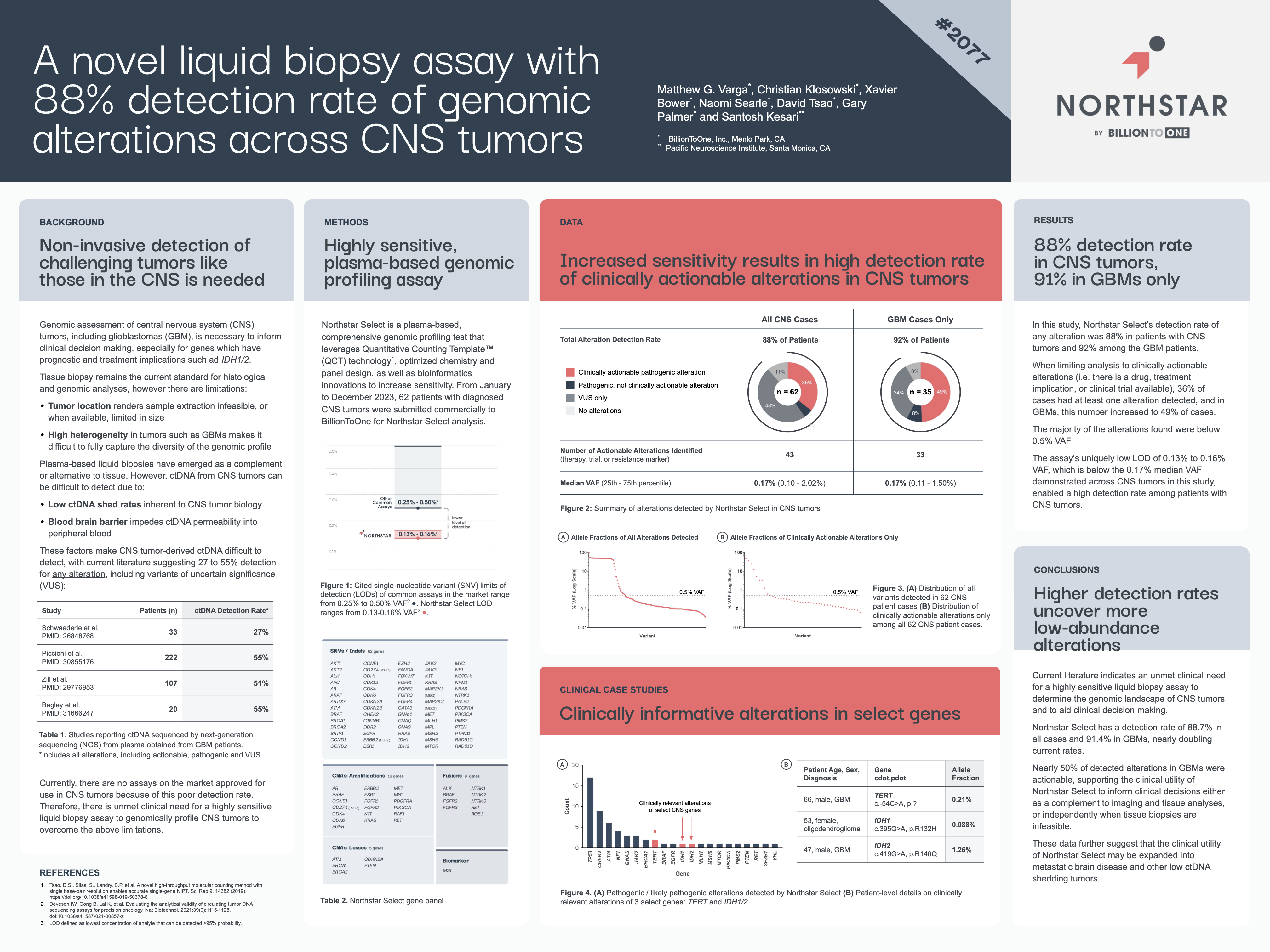
A novel liquid biopsy assay with an 88% detection rate of genomic alterations across CNS tumors.

Clinical validation of Northstar Select, a novel liquid biopsy assay for comprehensive genomic profiling of solid tumors.
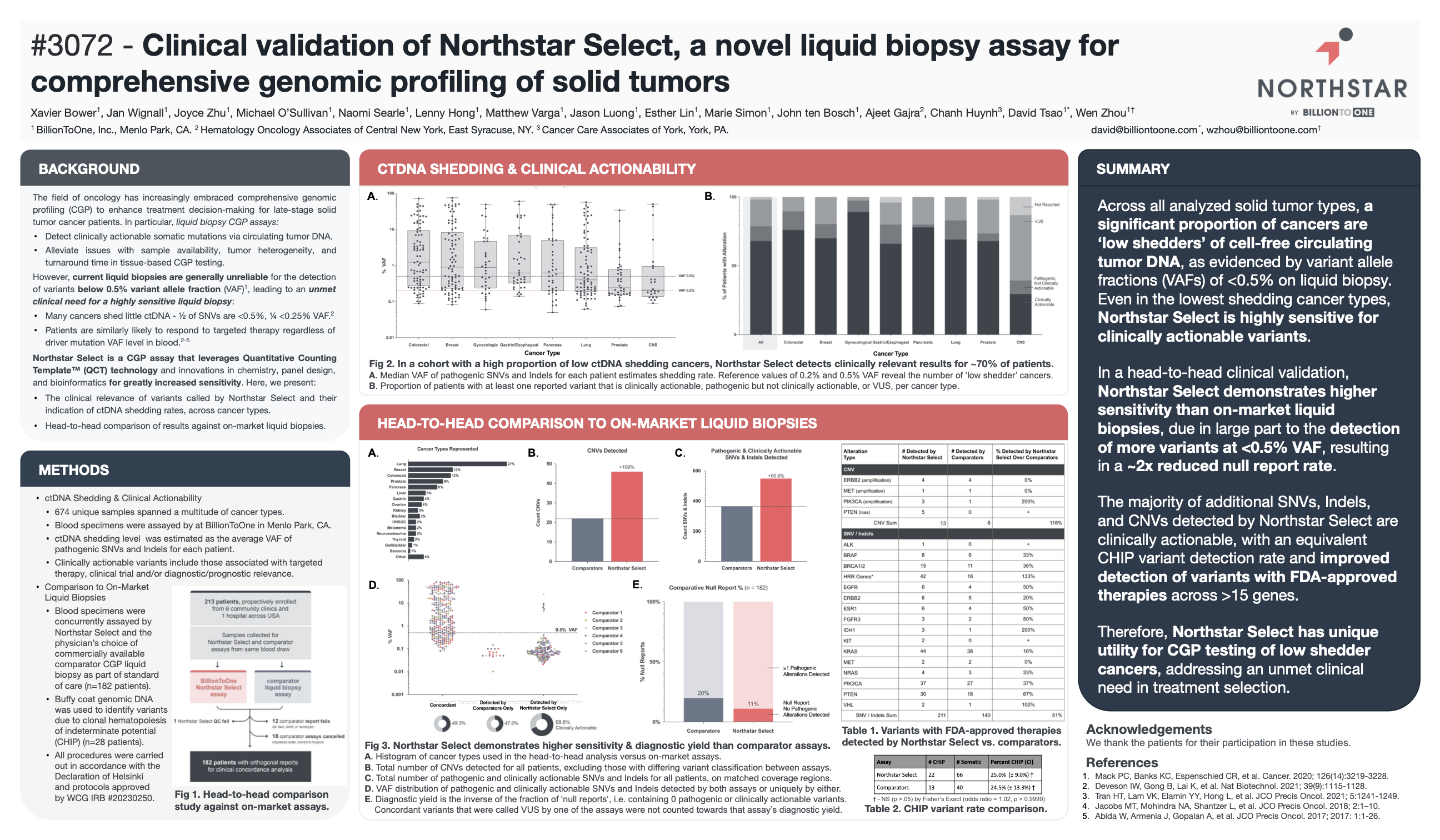
Clinical validation of Northstar Select, a novel liquid biopsy assay for comprehensive genomic profiling of solid tumors.

Analytical and clinical validity of Northstar Select, a quantitatively enhanced liquid biopsy assay for comprehensive genomic profiling of solid tumors

Analytical and clinical validity of Northstar Select, a quantitatively enhanced liquid biopsy assay for comprehensive genomic profiling of solid tumors

Clinical Validation of Northstar Response, a novel methylated ctDNA therapy response monitoring assay in patients with advanced GI cancer undergoing active treatment

Clinical Validation of Northstar Response, a novel methylated ctDNA therapy response monitoring assay in patients with advanced GI cancer undergoing active treatment

Methylated ctDNA as a biomarker for detecting previously undetectable clinically significant somatic mutations.

Methylated ctDNA as a biomarker for detecting previously undetectable clinically significant somatic mutations.