Northstar Response®

Adapt cancer treatment planning in real-time.
Analytical limit
of detection
Targeted
methylation loci
Northstar Response® is a tissue-free, methylation-based assay that enables real-time monitoring with single-molecule precision, detecting subtle shifts in tumor burden at sensitivity down to 0.01% tumor fraction.
Cancer doesn't wait. You need an assay that can stay ahead.
Enhance treatment monitoring with real-time tumor tracking and epigenomic insights from a simple blood draw, enabling earlier, more confident decisions.
Pan-cancer and treatment agnostic response monitoring
Assess epigenomic changes from over 2200 cancer-specific methylation sites
Limit of detection down to 0.01%1 TF for extreme sensitivity, with tissue-free convenience
Quantifiable and precise Tumor Methylation Score (TMS) at every collection
WBC noise filtering: incorporates a sequencing step to remove background DNA methylation from white blood cells found in the buffy coat
Applicable for all late-stage cancer patients on any treatment regimens
More timely insights to optimize treatment strategy
Imaging remains a key tool but it often lacks the precision or timing needed for real-time decisions. Northstar Response complements imaging by delivering early, actionable signals at the single-molecule level — often weeks in advance.
Monitor between scans
Get real-time feedback on therapy effectiveness without waiting for the next imaging cycle — helping you stay one step ahead.
Clarify imaging results
Use molecular signals to support decision-making when imaging results are unclear or confounded by pseudo-progression or inflammation.
Adapt to changes sooner
Detect subtle changes in tumor burden earlier than traditional tools, allowing for more timely treatment adjustments or escalation.

The only tissue-free treatment monitoring test that achieves single-molecule precision.
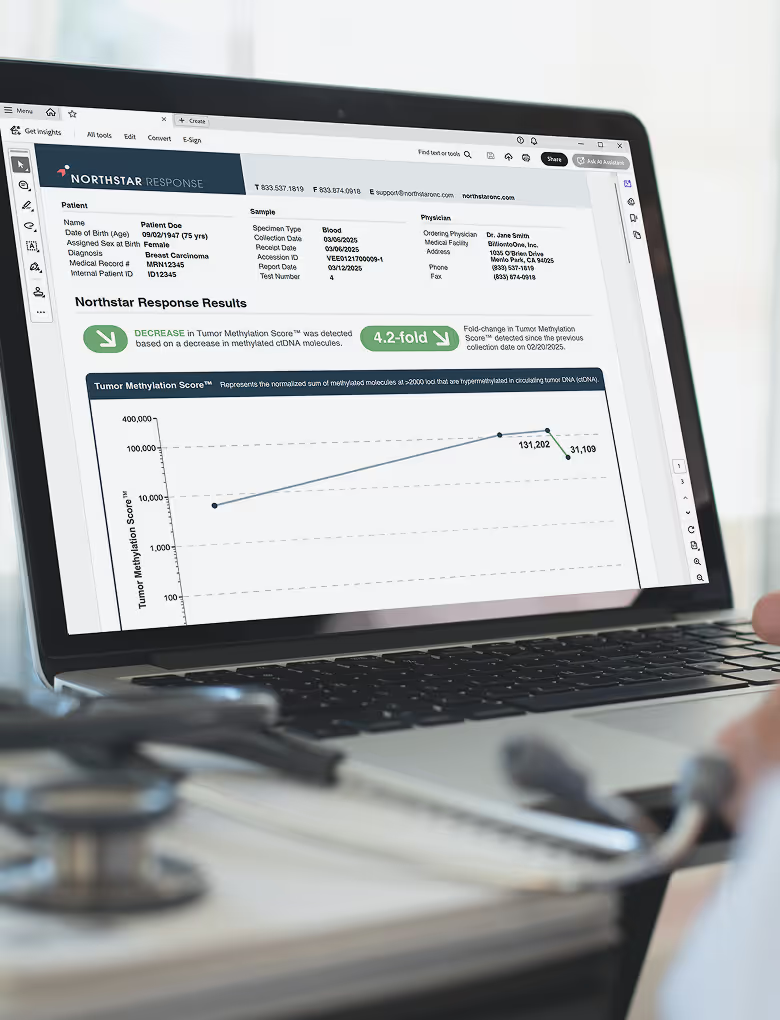
The Northstar Response report was designed to easily convey longitudinal monitoring tumor burden trends
for patients on systemic treatment (chemotherapy, immunotherapy, targeted therapy).
Clear result indicators: categorizes whether tumor burden increased, decreased, or remained stable
Precise Tumor Methylation Score™ (TMS): quantifies tumor burden in each sample
Graphical TMS trend: Easily identify trends between collections

About Tumor Methylation
Versus SNV- / Mutation-Based Monitoring Tests
Tumor methylation is a robust, tumor-specific biomarker2,3 that overcomes the limitations of tracking only variant allele fractions (VAF) from a few somatic mutations, as in first-generation ctDNA assays.
As a liquid-only test, it requires no tissue and avoids the delays and complexity of tumor-informed approaches, delivering faster insights into treatment response.

Download Helpful Resources
FAQ for Northstar Response®
Analytical validation data on file
Ye, PP., et al. Molecular counting enables accurate and precise quantification of methylated ctDNA for tumor-naive cancer therapy response monitoring. Sci Rep. 2025 Feb 18;15(1):5869.
Hsiao, A., et al. Brief Report: Methylation-Based ctDNA Serial Monitoring Correlates With Immunotherapy Response in NSCLC. Clin Lung Cancer. 2025 Jan;26(1):72-77.
