Northstar Biopharma Solutions
Precision without compromise.
Powering research from blood.


Unlock more from every drop of blood.
At BillionToOne, we build ultra-sensitive, liquid-only oncology assays that expand and accelerate what’s possible in biomarker driven drug development.
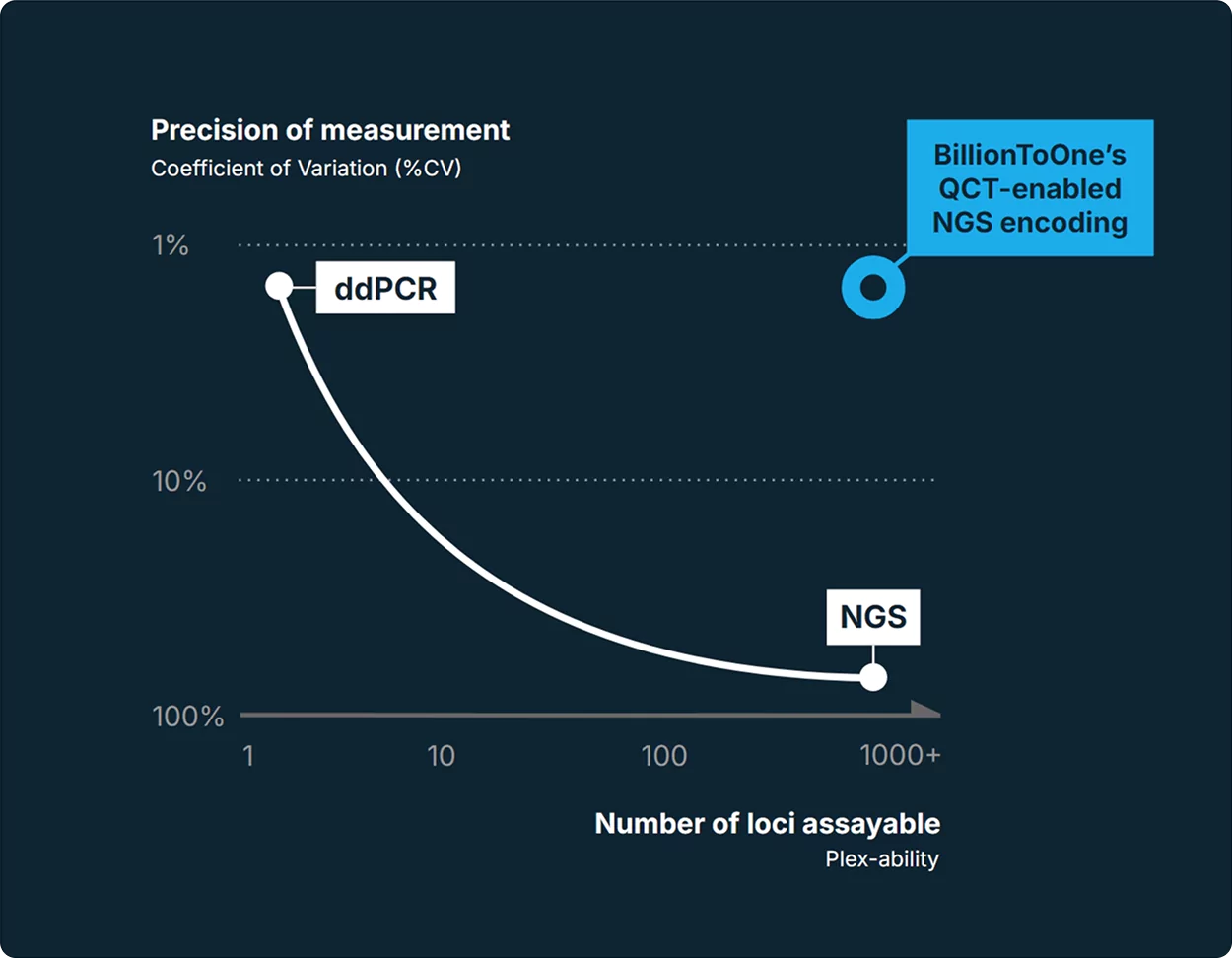
Our proprietary Quantitative Counting Templates™ (QCT) technology unlocks single-molecule precision from ctDNA—allowing you to detect variants and signals that other tests miss.
Our Biopharma Platform Supports:
Clinical Development
Accelerate pivotal trials with sensitive, non-invasive tools for patient selection and real-time monitoring — enabling faster enrollment and reducing time to trial completion.
Translational Research
Explore tumor biology and optimize variant identification of low frequency mutations or in low-shedding tumors.
CDx Programs
With ultra-sensitive liquid biopsy and a scalable platform, Northstar supports confident development from early-phase expansion.
Our Oncology Assays
Northstar Response®
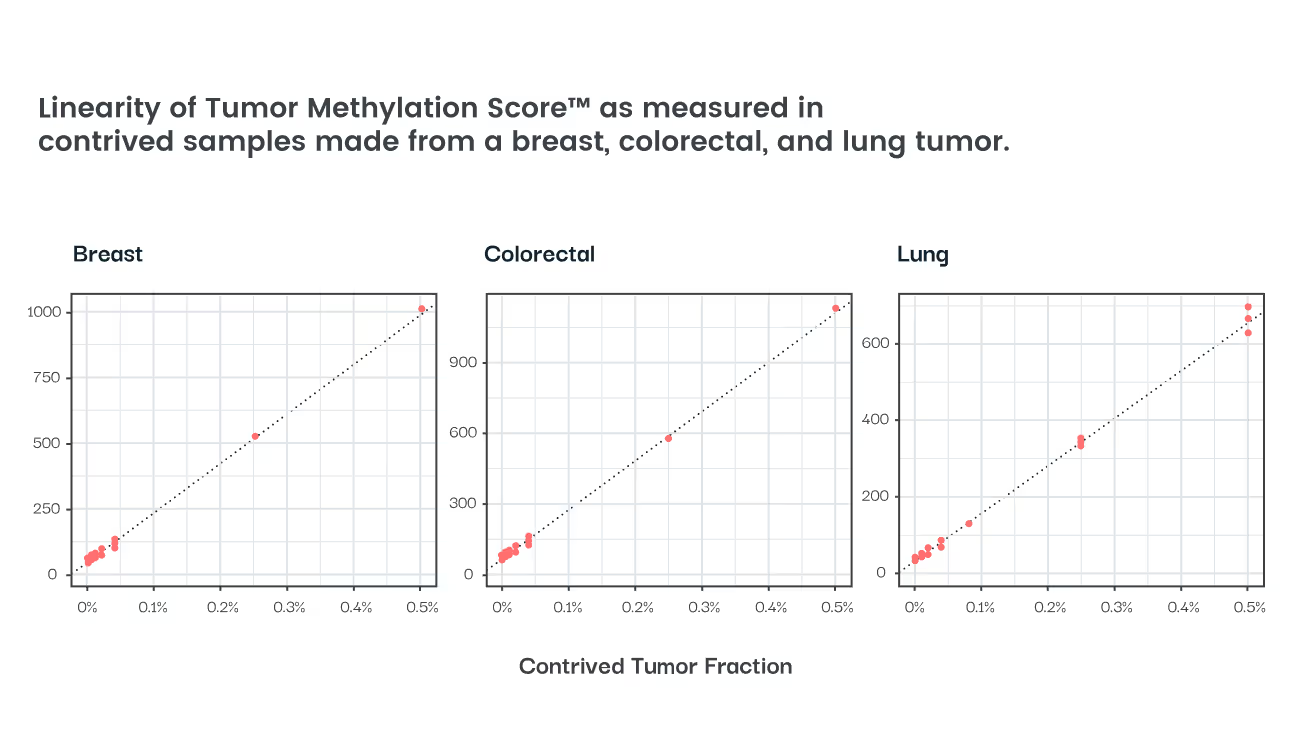
A tissue-naïve, methylation-based ctDNA assay with single molecule precision for real-time treatment response monitoring through epigenomics.
Assay Highlights3
>2200 cancer related loci, methylation based ctDNA signature
LOD = 0.01% tumor fraction
Single-molecule level resolution powered by QCT™
Sub-clonal methylation pattern change provides additional insights into tumor evolution
Northstar Select®
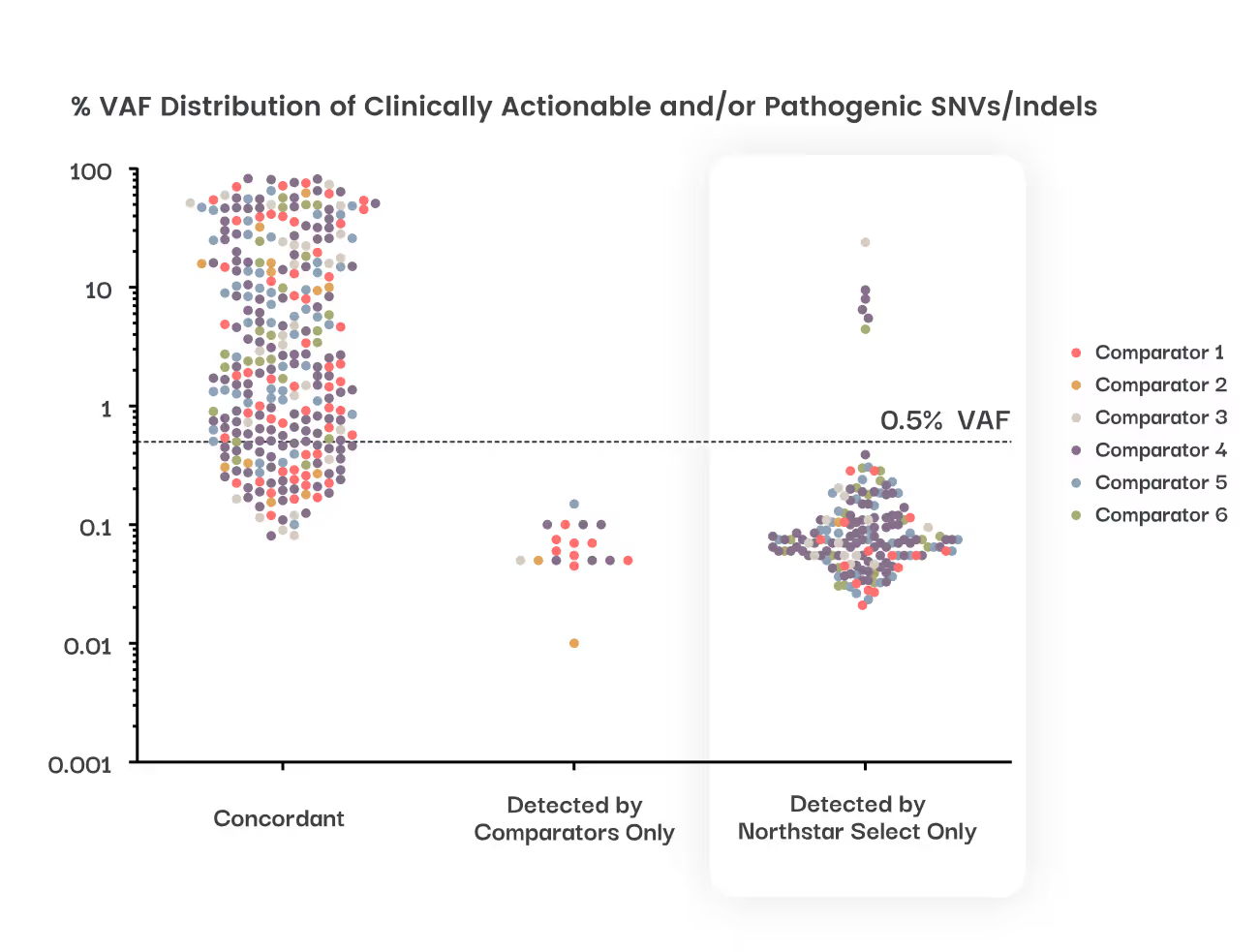
The most sensitive tissue-free liquid CGP assay, detecting SNVs, indels, CNAs (amplifications and losses), and fusions with 95% sensitivity down to 0.15% VAF1,2.
Assay Highlights
Detects up to 50% more variants from blood than other liquid CGP assays in a landmark head-to-head study
Unmatched CNA calls for amplifications or losses
Determines focal vs. aneuploidy amplification
Reduces false-negative reports by half
Work with us to accelerate your R&D portfolio
Based on a head-to-head clinical study [Bower, X., et al., JCO 42, 3072-3072(2024)].
*LOD 95% (Limit of Detection 95%) is defined as the lowest concentration of an analyte in a sample that can be consistently detected with ≥95% probability. Comparator assays have a LOD 95% that ranges from 0.20% -0.40%.
Analytical validation data on file
